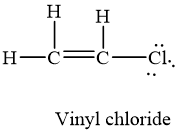
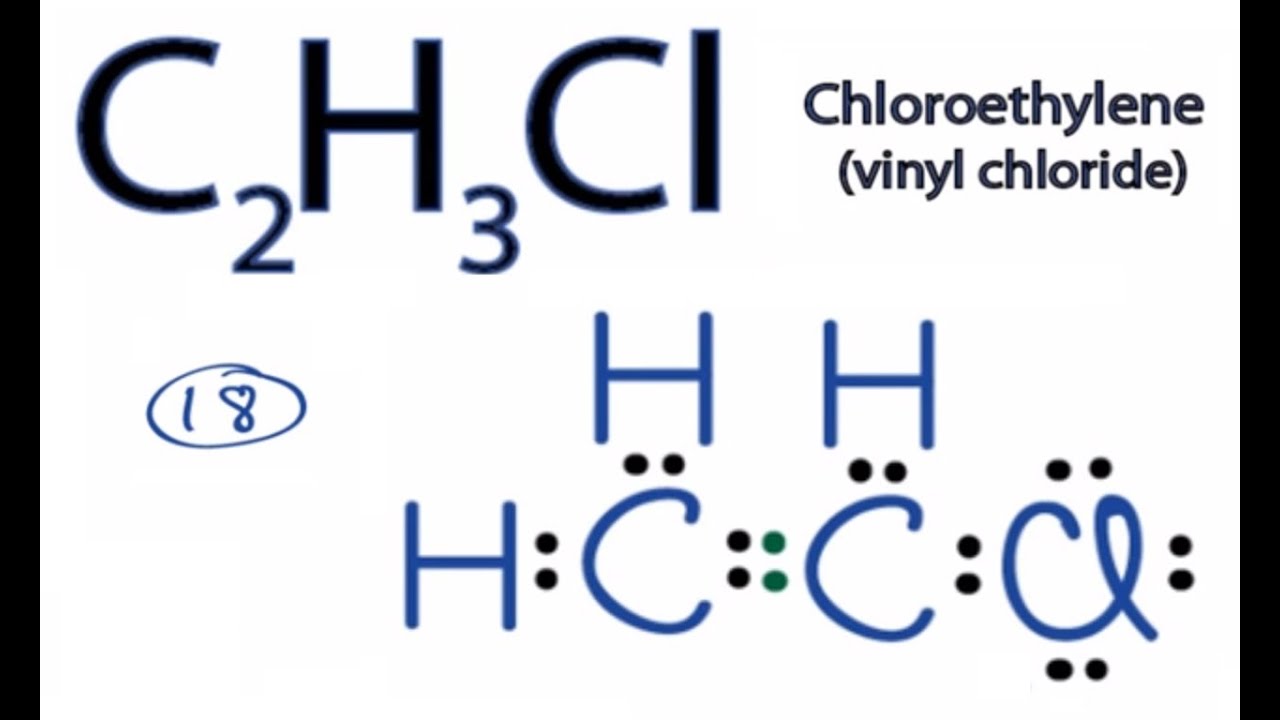
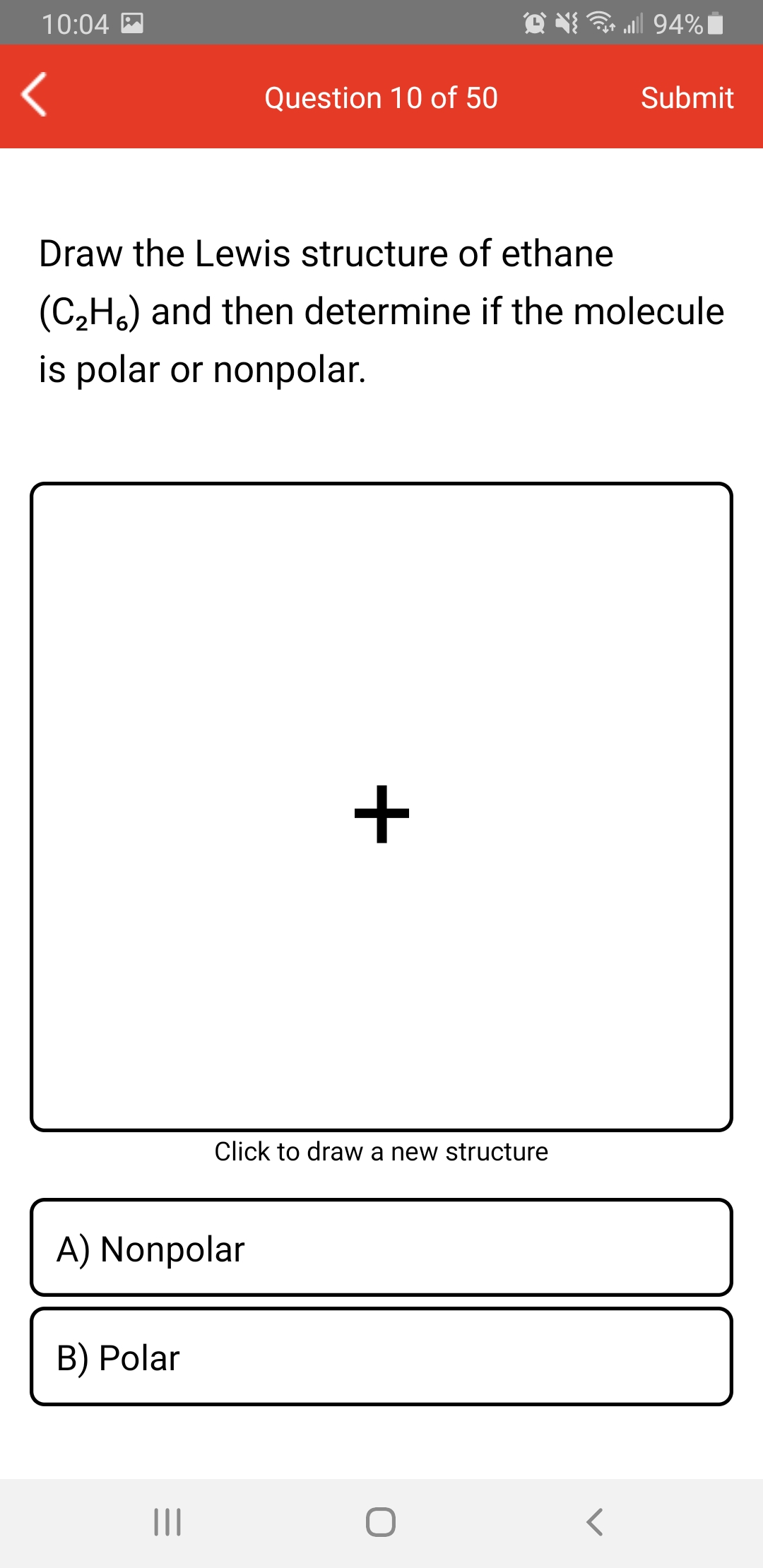
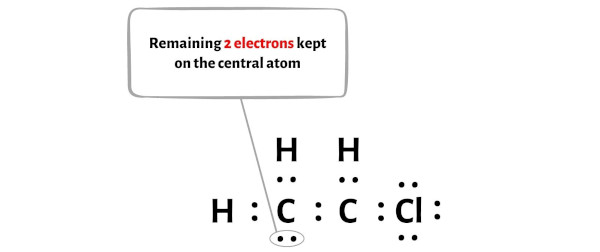
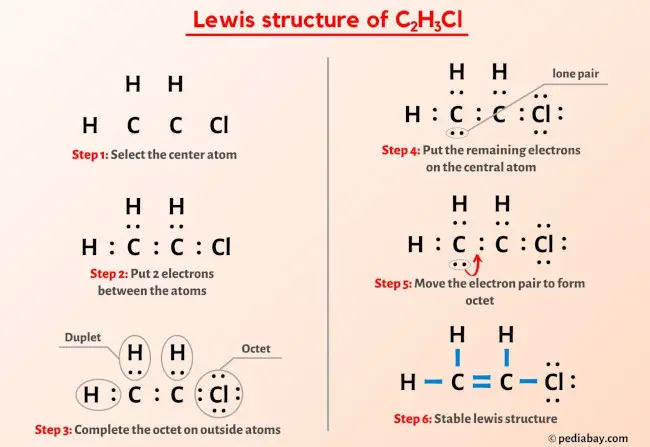
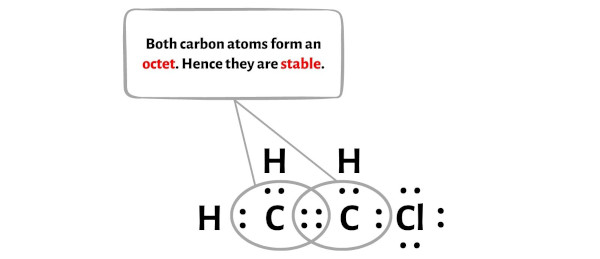
Draw a Lewis structure for C2H3Cl Show all unshared electron pairs None of the atoms bears a forma - YouTube

C2H3Cl Lewis Structure (Chloroethylene) | C2H3Cl Lewis Structure (Chloroethylene) C2H3Cl is a chemical formula for Calcium chloride. Check out this video to know what we do differently to... | By Geometry Of

Using an arrow, indicate the overall direction of the dipole moment of C2H3Cl3. Determine if there is more than one isomer for the given molecular formula. If so, draw them. | Homework.Study.com

Biacetyl and acetoin are added to margarine to make it taste more like butter. Complete the Lewis structures, predict values for all C-C-O bond angles, and give the hybridization of all the
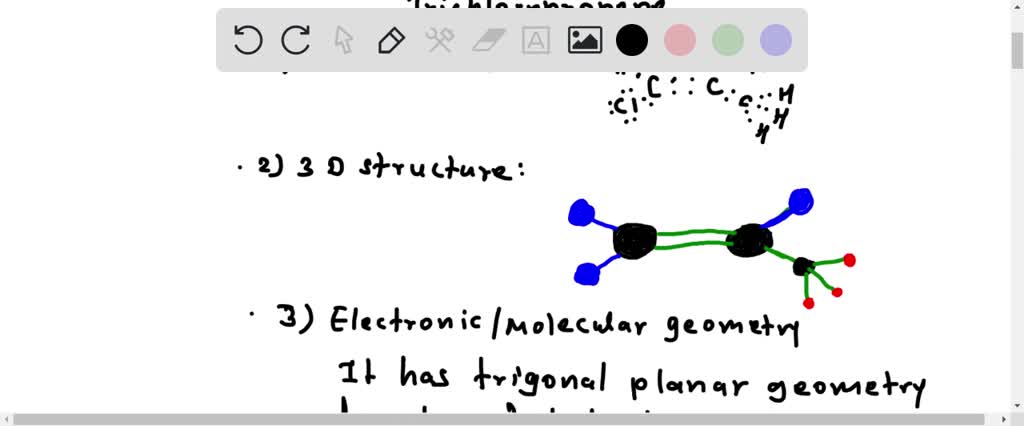
SOLVED: Draw and write C2H3Cl3 1. Lewis Structure 2. 3D structure 3.Electronic Geometry / Molecular Geometry 4.Hybridization 5. Polar or Non-polar
A student places a mixture of plastic beads consisting of polypropylene (PP) and polyvinyl chloride (PVC) in a 1.0 L beaker cont